The BPES full product catalogue

Cell Imaging & Analysis
SYNENTEC NyONE Compact Cell Imager
Bioreactors & Fermenters
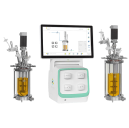
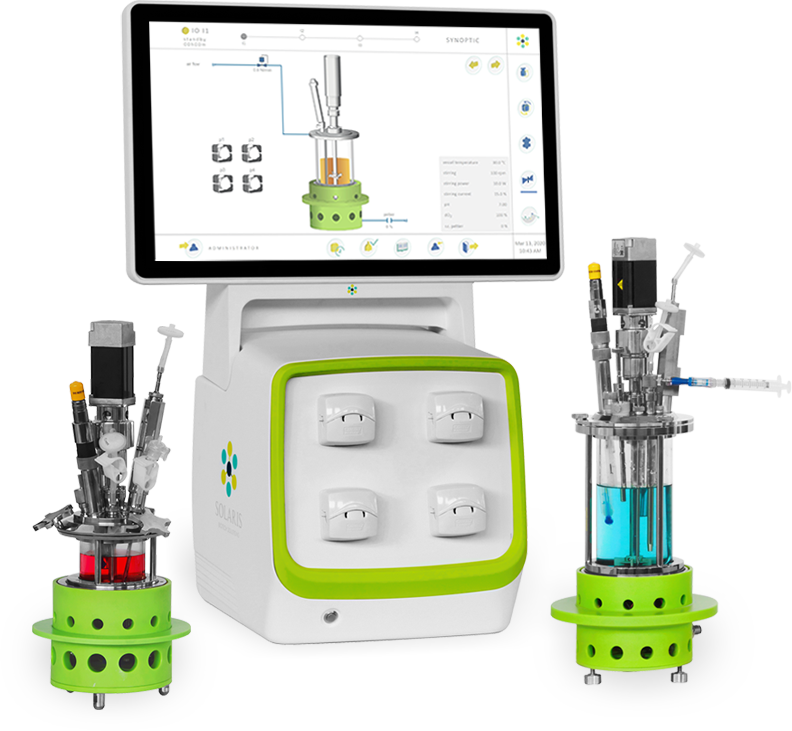
Solaris IO 200-1000mL – Benchtop Mini Bioreactors & Fermenters
Shake Flasks and Consumables
Thomson Ultra Yield Flask – 125mL to 2.5L
Biotech Pumps
Quattroflow QF30SU Single-Use Biotech Pump
Cell Disruptors
One Shot Cell Disruptor 0.5mL to 8mL
Bioreactors & Fermenters
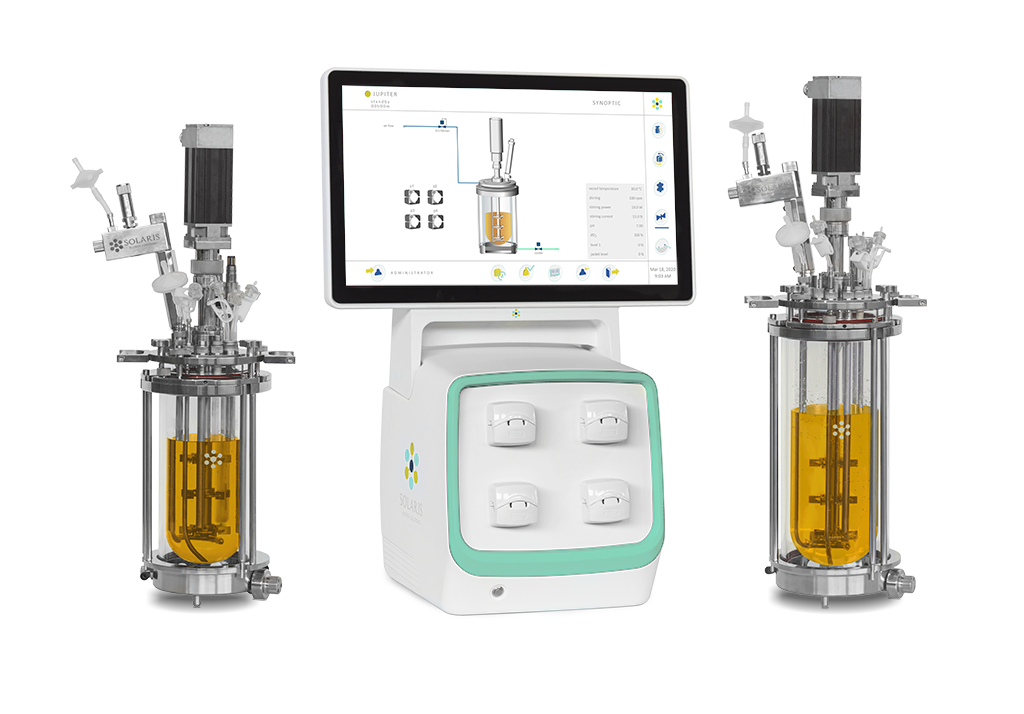
Solaris Jupiter 2-10L – Autoclavable Benchtop Fermenters & Bioreactors
Biotech Pumps
Quattroflow QF150 Biotech Pump
Cell Disruptors
Multi Cycle Cell Disruptor 0.5mL to 80mL
Shake Flasks and Consumables
Thomson Optimum Growth Flask – 125mL to 7L
Biotech Pumps
Quattroflow QF1200 Biotech Pump
Cell Disruptors
Continuous Flow Cell Disruptors up to 6L/hr & 24L/hr
Shake Flasks and Consumables
Thomson Sampling Flasks
Bioreactors & Fermenters
Solaris ONE – Single Wall Fermenters / Bioreactors
Cell Disruptors
High Flow Rate Cell Disruptor up to 150L per hour
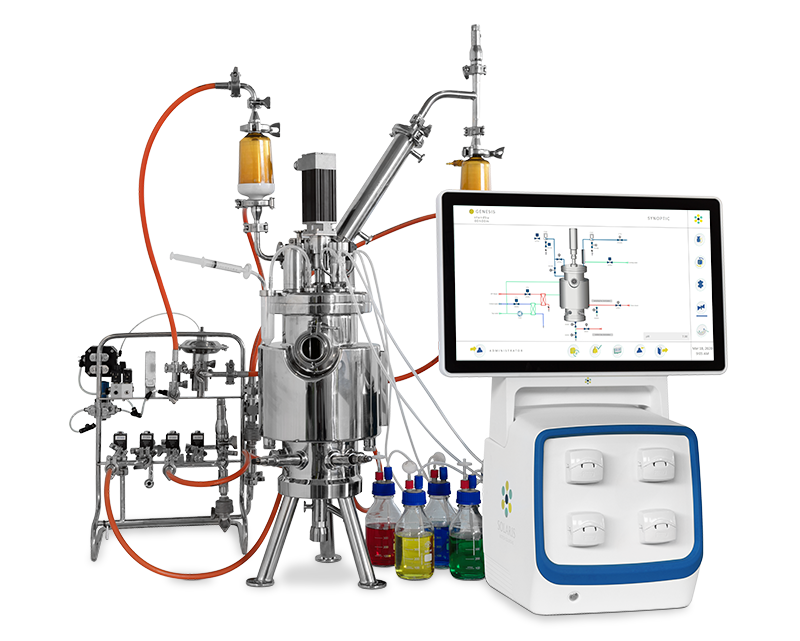
Bioreactors & Fermenters
Solaris Genesis 7.5-20L – SIP Benchtop Bioreactor & Fermenter
Shake Flasks and Consumables
Thomson Multiport Flasks